
Antiviral and Antibacterial-treated Leather
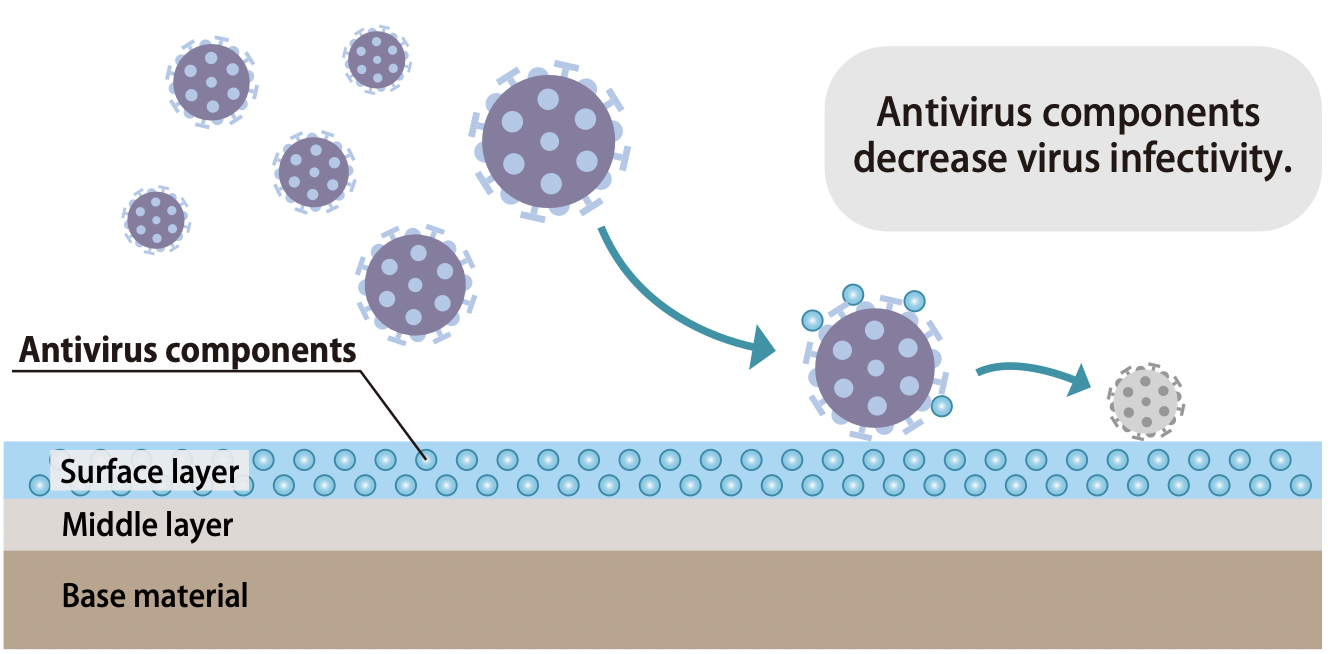
Virus Reduction Mechanism
SIAA
A first in Japan! In August 2020, we became certified for antiviral genuine leather surface materials by the SIAA *1. We have also become certified for antiviral synthetic leather materials by the SIAA.
The SIAA mark is granted to products that pass the ISO 21702 test, and their quality is managed and information is disclosed according to the guidelines of the Society of International sustaining growth for Antimicrobial Articles.
Caution!
- Antiviral treatments are not intended to treat, cure, or prevent disease.
- Our product complies with the SIAA safety standards.

High Antiviral Performance
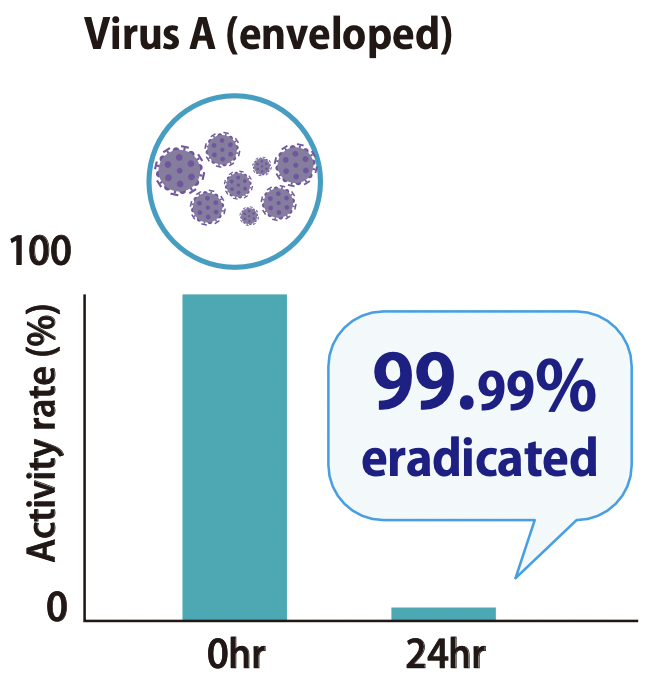
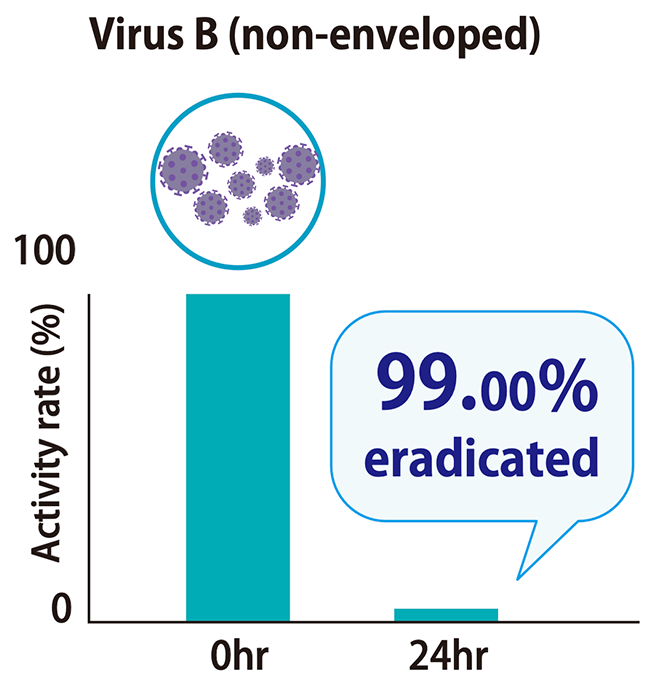
Testing laboratory: Nissenken Quality Evaluation Center Measurement method: Plaque assay Test method: ISO 21702
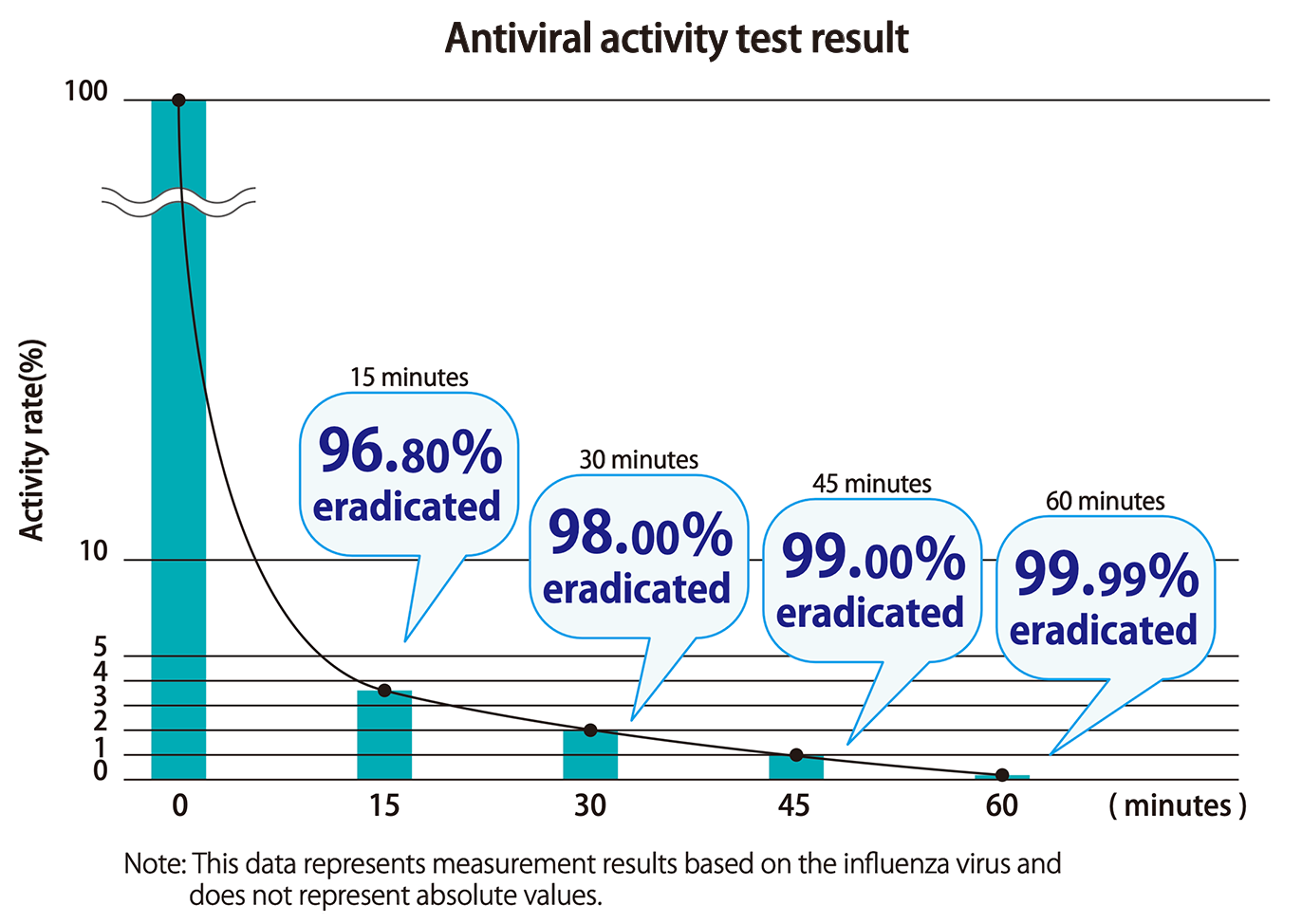
Immediate effect
The time from exposure to the virus to onset of effect and degree of effectiveness were verified. It was confirmed that 99.99% eradication was achieved at 60 minutes.
Testing laboratory: Biomedical Science Association
Measurement method: Plaque assay
Test method: ISO21702
Durability (duration)
Surface abrasion resistance tests were conducted (assuming 5 years of automobile service life) and X-ray analysis was used to verify the presence of antiviral components. It was confirmed that antiviral component levels remained consistent throughout the test, and that antiviral properties were maintained over the long term.
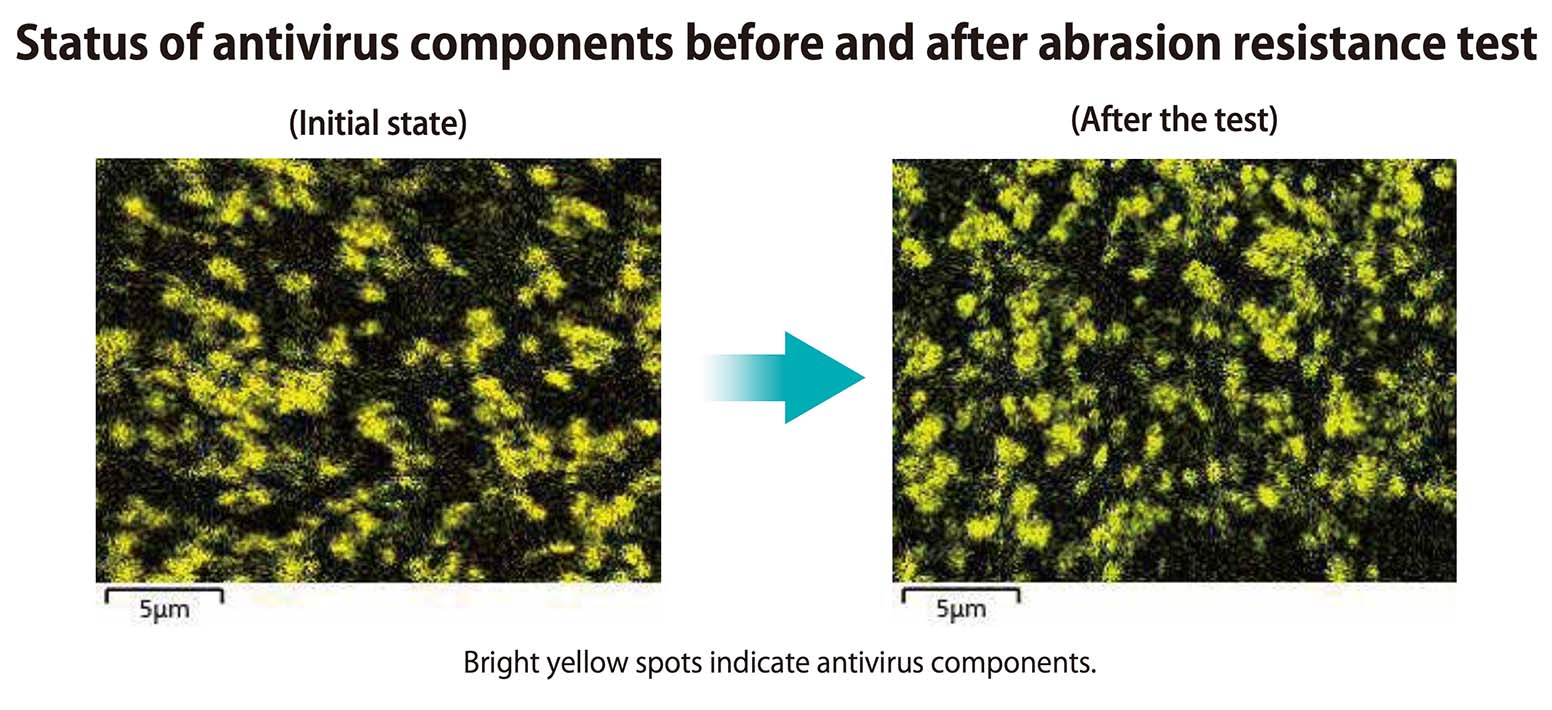
Note: Analysis conducted using X-ray
microanalysis with an electron microscope
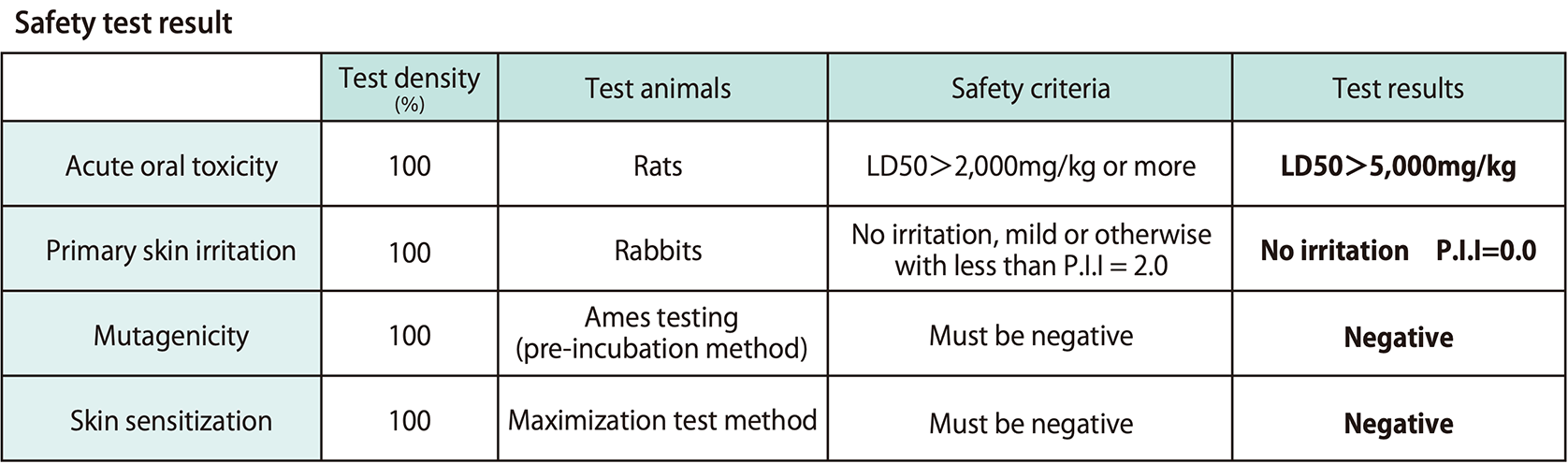
Safety
Our antiviral and antibacterial agents are comprised of ingredients considered safe for the body. These agents have passed the following third-party safety testing.
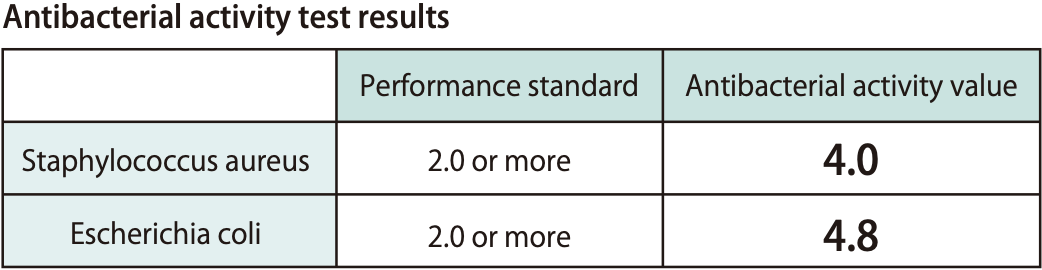
Antibacterial Activity
Our antiviral and antibacterial agents are comprised of ingredients considered safe for the body. These agents have passed the following third-party safety testing.
Testing laboratory: M.I.C. Co., Ltd.
Test method: JIS Z 2801:2010 (ISO 22196:2007)
